The Benefits of QMS Document Management System
Implementing a Quality Management System (QMS) document management system is crucial for organizations looking to streamline their processes, ensure compliance, and maintain high standards of quality. A QMS document management system provides a centralized platform for managing all documents related to quality control, audits, procedures, and more.
Here are some key benefits of utilizing a QMS document management system:
- Centralized Document Repository: All quality-related documents are stored in one central location, making it easy to access and manage important information.
- Improved Version Control: With a QMS document management system, you can track changes to documents, maintain version control, and ensure that the most up-to-date information is always available.
- Enhanced Collaboration: Teams can collaborate on documents in real-time, share feedback, and work together to improve processes and procedures.
- Compliance and Audit Readiness: By organizing all quality-related documents in a systematic manner, organizations can easily demonstrate compliance with industry regulations and be prepared for audits.
- Increased Efficiency: Automating document workflows and approval processes can significantly reduce manual tasks, saving time and improving overall efficiency.
A QMS document management system is not only a tool for organizing documents but also a strategic asset that helps organizations uphold quality standards, drive continuous improvement initiatives, and achieve operational excellence. By investing in a robust QMS document management system, organizations can optimize their quality control processes and set themselves up for long-term success.
Understanding QMS Document Management: Key Concepts and Examples
- What are the 4 levels of documentation in QMS?
- What is QMS and how does it work?
- What is quality document management system?
- What is an example of a QMS system?
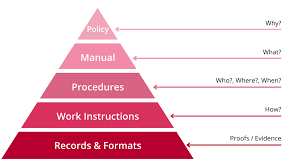
What are the 4 levels of documentation in QMS?
In a Quality Management System (QMS), documentation plays a crucial role in ensuring transparency, consistency, and compliance with quality standards. The 4 levels of documentation in QMS are essential for establishing a structured approach to managing quality-related information. These levels typically include policy documents that outline the organization’s commitment to quality, procedure documents that detail specific processes and workflows, work instruction documents that provide step-by-step guidance for tasks, and records that document evidence of compliance and performance. Each level of documentation serves a distinct purpose in maintaining the integrity of the QMS and supporting continuous improvement efforts within an organization.
What is QMS and how does it work?
A Quality Management System (QMS) is a structured framework designed to manage and improve the quality of products, services, and processes within an organization. It encompasses a set of policies, procedures, and processes that ensure that products and services meet defined quality standards and customer requirements. A QMS works by establishing clear quality objectives, implementing standardized processes for quality control and assurance, monitoring performance metrics to track progress, and continuously seeking opportunities for improvement. By systematically managing quality throughout all aspects of an organization, a QMS helps enhance customer satisfaction, reduce risks, increase operational efficiency, and drive overall business success.
What is quality document management system?
A Quality Document Management System, often referred to as QMS Document Management System, is a specialized software solution designed to centralize and streamline the management of quality-related documents within an organization. It serves as a comprehensive platform for storing, organizing, accessing, and controlling documents such as quality manuals, procedures, work instructions, audits, and compliance records. By utilizing a Quality Document Management System, organizations can ensure that critical quality documents are easily accessible, up-to-date, and compliant with industry standards and regulations. This system plays a vital role in enhancing document control processes, facilitating collaboration among teams, improving efficiency in managing quality documentation, and ultimately supporting the organization’s commitment to maintaining high-quality standards across all operations.
What is an example of a QMS system?
An example of a Quality Management System (QMS) system is ISO 9001, which is an internationally recognized standard that sets out the criteria for a QMS. ISO 9001 helps organizations establish processes to ensure consistent product quality, customer satisfaction, and continuous improvement. By implementing ISO 9001, companies can demonstrate their commitment to meeting customer requirements, complying with regulations, and enhancing overall operational efficiency. This QMS system provides a framework for organizations to create a culture of quality excellence and drive sustainable business growth.
